Mass spectrometry is a versatile tool to analyze the elements present in the samples as well as to estimate their concentration. Mass spectrometer is the instrument used for atomic mass spectroscopy.
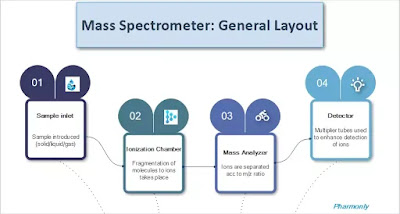
Mass spectrometer simply composes of four components. Sample inlet is the first component through which the sample proceeds further in
mass spectrometer. The pressure inside sample inlet is lower than that of lab
environment.
The sample vapours then proceeds to the ionization chamber
in the form of fine beam of molecules where they get ionized by a high energy
beam of electrons. The ions formed here
are accelerated to mass analyzer.
Mass analyzer separates the ions based on their m/z ratio with the application
of magnetic field. Different types of mass analyzers are there according to
their resolution and uses.
The ions separated in the analyzer are detected in detector.
Electron multipliers are used for rapid and accurate detection. The result in
the form of mass spectrum is plotted on data system.
One term going to be used throughout the topic is mass to
charge (m/z) ratio of atomic or molecular ion. Mass to the charge (m/z) ratio
of an ion is the ratio of its mass number to the number of fundamental charges
z on the ion.
For example; 12C1H4+ has the m/z ratio = 16.0313/1 = 16.0313 And 13C1H42+ has m/z ratio = 17.0346/2 = 8.5173
One should remember that the mass of ion calculated by the
mass spectrometer is the true mass of the particular ion i.e. the sum of masses
of atoms in single ion. The mass value obtained from chemical atomic weights is
slight different from it as the chemical scale of atomic weights is based upon
the weighted averages of the weight of all the isotopes of the particular
element .



0 Comments
Let me know your valuable suggestions and queries here.